Saturday, December 03, 2005
Feeling holier than thou?
Yeah, I suppose it seems that way at times. But now I'm actually feeling pretty good about the Firm, and I'll explain why.
I recently attended a forum where members of my department gave feedback to management about all sorts of issues, about which our management was found lacking, in one of those bi-annual workplace satisfaction surveys that my company conducts for all employees.
During this forum, I had a sidebar discussion with a physician, about the safety review process at our company. This is where all of our safety data is reviewed annually for each drug, and changes are made to the drug's labeling. He said that our company was the first he's worked at where there was no commercial input in this process. It is left entirely to the safety physicians to determine the contents of the safety sections of the labels. He had worked at four other companies, and singled out Wyeth as one where the sales organization had the largest impact on the safety review process. We have several other Wyeth alumni from the mid 90s, and I asked them how much the sales organization affected the safety group back then. They all just smiled, shook their heads gently, and walked away. I wonder why?
Tuesday, November 01, 2005
Economic models, and how we work
Today, my department received a presentation on a new model for international cooperation and harmonization of processes, raising issues, etc. We have three major sites worldwide in my group, and several satellite sites as well.
Just before this presentation, we were given an overview of the Companies efforts to promote Medicare Part D, by an individual from our government affairs office. All employees are expected to have at least minimal knowledge of the program, in anticipation of questions from family and friends that may come our way. To his credit, he was very upfront about the benefit to patients and the company by having hundreds of different plans compete for patients. Better to have hundreds of formularies to try to influence, than an all or nothing government formulary, was his message.
The contrast between these two presentations was thought-provoking. The Medicare presenter was part of the "commercial" organization, very articulate and polished, with a very persuasive message about the benefit of Part D to our company's bottom line. It's brilliant how patients' needs and the Company's benefits are perfectly aligned in so many issues. It's a great PR strategy, to always appear to be on the side of the patient, whether it be promoting "choice" Medicare coverage, or patient safety from counterfeit drugs (remember all those dying Canadians!) or on many other issues. More on that another day.
But the message about our department operating model was starkly different, in a political/philosophical way. Frankly, it was pure socialism in its design. It was all about centralized planning and control of processes, so groups or sites don't get too independent. It was stressed that we need to know how all the areas are operating, in light of FDA inspections that can occur anywhere in the world.
In a decentralized, capitalistic model of our department, we would all be given our goals for the year for output, and told to reach (or preferably, exceed) them in any way we saw fit, provided we didn't breach any laws. And I could not imagine anyone I work with would support such a model. This is a company and industry with an overwhelming (but not exclusive) alliance with Republicanism. But free market thinking just doesn't exist within my workplace.
Friday, October 28, 2005
Stranger than Fiction?
The Puffington Host already had this, and Sploid picked it up from there, but I don't know if all of my reader(s) would see it there. In short, a guy was hired by PhRMA to write a thriller about adulterated drugs from Canada, poisoned by nasty swarthy types. The plug was pulled, and PhRMA's management claims ignorance about this project, set up by a "lower-level employee who acted without authority." The juicy part at the end of this? The book is coming out anyway, supposedly with a drug company behind the poisoning conspiracy! Bitchin! Pre-order today!
The plot thickens....
A reader asked me about possible plot holes in "The Constant Gardener," and I'll try to reply. How can a company hope to market a drug that quickly maims or kills a number of patients? The answer depends on what the drug is for - how deadly is the disease you're trying to cure? Specifically, in the movie the drug is being used for tuberculosis, and there are hints that the drug just needs "fine-tuning" before it can be submitted to regulatory authorities for approval. This is only conceivable if the "fine-tuning" has to do with finding the optimum dose range for the drug. You can't just muck about with the molecule, adding or removing an atom here or there to make it safer. Not yet, anyway. The closest I've seen to that sort of chemistry is to take an isomer of a drug, and to market it as a different compound, after testing. See this article for a good description, and some examples. An example of a film with an outlandish plot revolving around a bad drug, see "The Fugitive" where a drug ("Provasic" - great name!) to clear out blocked arteries was destroying patient livers. This drug would have been pulled within a few months after launch - it made no sense to hide such deadly effects during the trials, since the costs of pulling the drug off the market, with all the associated lawsuits and bad publicity, would be far more than just cutting your losses during clinical trials, and canceling the program. The truly paranoid just don't get that - we can't hide all the dead bodies, you know.
Wednesday, October 26, 2005
What...Me Worry??
Be Afraid...Be Very Afraid...of what?
A fabulous article that everyone should read appears in the NY Times today. Relative risk, folks. Thisarticle is for all of you idiots who stop using thesubway because you're afraid of terrorists, andinstead drive to work. Everyone I know needs a crashcourse on the statistics of harm (and lotteries too,but that's a topic for another day). Halloween isalso a good time to visit Snopes.com, not to mentionthe Department of Justice crime statistics. Acolleague of mine said today, "You just can't let kidsgo out trick-or-treating these days, like you couldwhen we were kids." Baloney. If it was safe ageneration ago, it's safe now. Our neighborhoods areno more infested with undesirables than they ever were- it's just that we can find out who they are, thanksto Megan's laws.
Monday, October 03, 2005
God bless the First Amendment
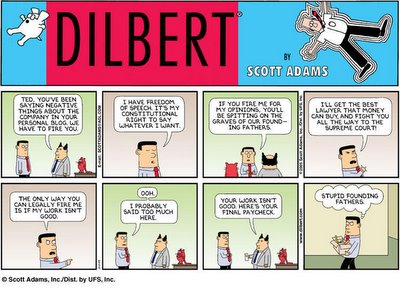
God, I am so screwed....(see above)
But I love my granny....
Merck has tried an interesting line of defense, though suitable only for public consumption. The Philadelphia Inquirer reported yesterday that several key executives have revealed that immediate family members of theirs took Vioxx, in an attempt to dissuade the casual non-thinker that the executives are, in the words of Opus, "Incompetent fibbing poopyheads."
This sort of thing is not admissable as a defense, because it assumes a) that Raymond Gilmartin did not intend to kill his wife, and b) that they didn't decide to take their chances just to avoid the $10 co-pay. In other words, there are lots of reasons someone might choose one drug over another.
Meanwhile, clean arteries.
The AP reports that the cardiologist who treated the plaintiff in the Atlantic City Vioxx trial had clean arteries, in the "top 5%." The stories do not mention who the physician was testifying for - the plaintiff, or the defense. This news indicates that the pool of possible plaintiffs might be smaller than just anyone who had a heart attack while on Vioxx, if they've also got some artery clogging. The goal of the plaintiffs is make Vioxx the proximate cause of the injury, and the evidence shows that it increases the risk of heart attacks, not plaque.
Thursday, September 29, 2005
Guidant is off-course
Guidant has been maufacturing cardiac resynchronization therapy (CRT) units, that stimulate different parts of the heart independently. Recently, they have recalled tens of thousands of units due to short-circuiting problems. See this article for some background. Of course, Guidant isn't new to safety fraud...
Seeding trials?
I don't know much about clinical trials for devices, and how much they differ from drug trials, but in one respect, there seems to be a striking similarity. This New York Times article describes a that Guidant allegedly practiced, using an "evaluation" trial as a method to spur sales. This is right out of the pharmaceutical playbook of old. The company paid physicians to fill out a survey about the devices, $1000 for five forms. Chump change for Guidant, it turns out, since the survey resulted in
"...>$2 million in new sales with physicians who are not necessarily GuidantThis is what used to be called a seeding trial - using a scientific pretext to generate sales, market share, buzz, or whatever.
friendly. We paid each physician who completed all five surveys $1,000 so
our
total cost was $80,000."
Several documents were sent to the Times by someone professing to be an employee of Guidant, but there is no mention of whether there was a protocol for this "evaluation" or whether there was any consultation with IRBs or ethics boards where the participating physicians practiced.
Kick back and read on....
But you can't pay physicians to use your product - that's plainly illegal. So these evaluations have to be considered research to avoid the federal anti-kickback laws. To avoid violating the law, the research purpose for which you pay the physician has to be legitimate. See this presentation to get a better idea of the requirements. To quote:
"Clinical trials or other research with little scientific value implicate theThe document then references the AMA definition of "Genuine Research Purpose" which you can find here.
federal Anti-kickback Statute and IRS requirements. In addition, clinical
trial proposals that offer inducements to physicians to participate implicate
the federal Anti-kickback Statute."
"How can a physician tell whether there is a "genuine research purpose?"The drug industry has developed, through their trade group PHRMA, a Code for Interactions with Healthcare Professionals. More recently, we have promulgated new standards for clinical trial conduct and communicating their results. The first document was a voluntary code for industry, but has now been incorporated into law, in the sense that the most recent federal regulations on anti-kickback for the drug industry specifically cites the Code for Interactions, considering them to be a minimum standard. We may see similar treatment for the clinical trial standards.
A number of factors can be considered. Signs that a genuine research purpose
exists include the facts that there are (1) a valid study protocol, (2)
recruitment of physicians with appropriate qualifications or expertise, and (3)
recruitment of an appropriate number of physicians in light of the number of
study participants needed for statistical evaluation."
At first blush, this may seem offensive. Since the industry wrote The Code, it sounds like the industry is therefore determining the law of the land for regulating themselves. I mean, who is supposed to write the laws? Should we have all industries write their own "Codes" and simply have the regulators defer to them?
However, we are persuaded that the PHRMA Code is actually not a bad starting point. In fact, we even heard Jim Sheehan, Associate US Attorney for the Eastern District of PA (Philadelphia) and frequent industry foe, refer to The Code as a good start, that fails only in that it does not address certain areas of conduct. In addition, by having The Code cited in the Federal Register, it makes adoption of this voluntary conduct a necessity to industry. No decent compliance or legal department would allow their sales folks to ignore it.
The Ectasy of Device Manufacturers
After a few more Guidant episodes, perhaps we'll see some similar reactions from the Medical Device Manufacturers Association (MDMA). A comparison between their website and PHRMA's website is instructive. PHRMA practices defensive medicine, knowing that it's an industry under siege. There are all kinds of articles and links to items showing how responsible and honorable they think they are. If we keep the pressure on, perhaps someday they'll live up to their own rhetoric.
Tuesday, September 27, 2005
Getting the Label
is this?. I might just have to take some Prozac.
Getting the Label
OK, let's talk a little about Vioxx. This is a pharmaceutical legal blog, and Vioxx is the big drug litigation story, so you'd think I'd spend more time on this.
Today, there's a telling AP story about Merck officials "getting the label" they wanted for Vioxx. The "label" is the package insert, which contains all the warnings and side effects about a drug, in addition to indications, pharmakinetics, chemistry information, etc. Getting the label you want for a drug is what clinical trials is all about - negotiating with the FDA, based on whatever trial data you have, to avoid warnings and precaution statements as much as possible. Negotiations sounds like a strange word in this setting, but it's what really happens. The drug company argues that there are too many confounding factors that prevent any assignment of causality. For a drug that is generally taken by sick people, this is an easier process for the company, since there is probably a substantial background rate of all kinds of ailments. For a pain drug, you have to consider the patient population who took the drug during the trials. Did they take it for arthritis pain, and are thus probably elderly? They probably take all kinds of other drugs then, with a whole bunch of side effects of their own. The VIGOR study was for rheumatoid arthritis, and if the patients were anything like my mom, they take a pharmacy every day.
In the big continuum that is drug development, before you even have a compound, you have a Target Profile. The profile tells you that you want a drug that does X, does not have side effect Y or Z (that the existing drugs in the therapeutic area have) and has a sales potential of $$$. Thus, you already have the beginnings of the label you want before you even have a compound.
I heard a lecture once from a drug safety physician in my company concerning a particular psychotropic medicine that had a warning about cataracts on the label, based only on animal studies. He had been told that getting rid of that warning was a high priority for drug safety, since it was responsible alone for losing hundreds of millions in sales annually. This was the first time that I heard a dollar figure attached to a safety issue, and it was clear that our marketing folks wanted us to go to the FDA with every argument possible to get rid of that warning.
So, the Merck folks came to the same conclusion about their drug, but they were more successful in their negotiations. They managed to convince the FDA to keep the heart attack information from VIGOR in the "Precautions" section of the label, instead of the "Warnings." This is significant not because the prescribers would notice the warning by themselves (they wouldn't), but rather the helpful competition would be sure to point it out to them, and feature it prominently in their sales materials. Imagine the script for a Pfizer rep - "Celebrex: the ONLY Cox-2 inhibitor without a heart attack warning."
The damning part of all of this is the attitude of the Merck players, as well as their stupid use of email. EMAILS LIVE FOREVER. Write them like they do. And remember - Merck was the most respected drug company in the country. If you asked a focus group about Merck two years ago, you'd get reactions like science, integrity, and so on. The evidence introduced in these trials should forever pierce that veil. How much of Scolnick's emails about VIGOR and the FDA had anything to do with patient well-being, do you suppose?
Tomorrow - Guidant was off-course!
Thursday, September 22, 2005
See what happens while I'm away??
Alright, fellow bloggers, how do I get this to stop? I posted about five minutes ago, and my inbox is getting filled with anonymous, clearly automated messages that read something like this:
Nice blog. Keep it up. I found this directory on How to drink and stay safe driving . I think it is a good thing that someone is educating us all on How to drink and stay safe driving. Simply PRICELESS!Imagine giving someone you love the opportunity to LIVE by giving them the proper stuff on How to drink and stay safe driving TODAY.
This is new since August - how can I make it stop?
Catching up with the news
But I wanted to focus this evening on issues that are away from the news. One has to do with my criticisms of Medicare Part D and pricing.
A reader criticized my writing about government drug pricing (even accusing me of being a lawyer - what a cheap shot!). He referred me to a book on economics, and briefly explained how government interference in pricing distorts markets.
He was completely correct about the interference of government, but was too limited in his critique of government as a purchasing agent. He reminded me (and I can't believe that I haven't already mentioned this to you) that the Feds require that pharmas sell to them at their lowest price. This is true, and does indeed distort markets. I remember when my company was concerned about their Indigent Patient Program - if we gave away drugs for a few bucks in co-pay to the poor, would that constitute a price? And the TogetherRX program required a waiver from the pricing policy as well, according to my reader.
I am for market pricing - I haven't complained to anyone about the recent gas prices, for example, and found merit in the Wall Street Journal article "In praise of price gouging" or whatever is was called. But there is much more distorting the price of pharmaceuticals than the VA supply schedule. The three largest contributors to price distortion are the patent system for drugs, the regulatory burden of pharmas, and the enormous scientific subsidies they receive.
I'm not going to argue for the abolition of patents or regulations. Hello 1902. No, instead I want to give some perspective to help explain why exempting Medicare Part D from any pricing regulations rubs me the wrong way. The factors that drive up pharma pricing are almost overwhelming, and call for a restraining hand. For example, there is no such thing as a start-up garage-based drug company. Pharma CEOs do not look over their shoulders at lean and hungry start-ups the way Bill Gates has to. Also, you can't look at a drug patent, figure out a quick way to come up with an approved product, and have it on the market in weeks or months. Finally, there are huge gaps between the consumer, the learned intermediary (the doctor) and whoever is paying for the drugs. For most consumer transactions, these three elements are the same.
Think about the impact these elements have on pricing. And think what a small counterweight it is to have regulated pricing for programs paid for with tax dollars.
I've Done This Before...
but I can't help myself. There was a large ad for this item in the coupon section of my Sunday paper a couple of weeks ago, and I almost snarfed my cornflakes. I think I've mentioned before that I know many people who work for McNeil Consumer Products, and it's fun to call them and tease about this. Five years ago, this would never have happened, or so my friends like to believe. Get a real drug company, guys.
Switcheroooooo
You had to know I would mention this item, about the atypical anti-psychotics versus perphenazine. It's important to keep in mind that the headline writers are missing a big point revealed by the studies. While perphenazine performed well, it had a large number of patients who had to discontinue, like the rest of the drugs. So a large armamentarium for anti-psychotics is more important than, say, adding another statin or non-sedating anti-histamine to the market.
By the way, I wouldn't lose too much sleep (har har!) if I worked at AstraZeneca, makers of the maligned Seroquel (maligned in this article, at least). The drug is the market leader in prescriptions, but not sales. Why? Because most of the use is for sedation ("Sleepoquel") at sub-therapeutic doses. Let's just hope they aren't promoting it that way. T'would be bad.
Gardening away
Yep, the movie was awesome. I also watched City of God recently, by the same director, and found it somewhat over-rated. But watch this space in December for shameless promotion of "The Constant Gardner" for Oscar consideration. I cried like three times.
Interesting note - check out this post on essentialdrugs.org, concerning the movie poster. Kind of bizarre. I was struck by the fact that Ralph Fiennes appears with an outstretched arm holding a gun on the movie poster, which never occurs in the film. Also, in the movie's trailer, there's clearly a scene that was set in a snowy area, presumably in Western Canada, where part of the book is set. This is also not in the film.
Saturday, August 27, 2005
Morning After Post
Focus on Medicare
I got to take a little break from my cube and computer last week to participate in a focus group on Medicare Part D. It was the second time I had been invited to do so in my Company, and I was eager to voice my opinion. This time, we were being asked to evaluate educational materials for both patients and employees. The idea is that we as employees may be asked by patients what it's all about, and we should be knowledgable.
But first, we got to sound off on the program as a whole, and my fellow employees did not let me down. No myrmidons we, many negative opinions were expressed. I spoke about how the complexity of the program was a direct result of our lobbying against any kind of single payer system, while others worried about the liability of Medicare, especially after I pointed out that it will be insolvent far sooner than Social Security. The Public Affairs guys and gals in the back of the room took notes. I don't know if they were expecting this.
I have not missed an opportunity to say something about Part D whenever I could, and truthfully, I've never felt uncomfortable expressing anti-industry opinions in forums such as this. My company has one of the lowest rates of employee contribution to PACs in the industry, so I like to think that there's a lot of independent thinking going on here.
Still I remain vigilant. I've heard people complain about Medicare Part D in terms of its complexity - it's usually something like "God---ned government can't do anything right. Why do they make my mother/father/relative shop around for these ridiculous cards?" I don't hesitate (and neither should you, loyal reader(s)) to enlighten that person. It was us. We caused it to be like that. Without those cards, without the shopping around, without the explicit prohibition of government price setting like the Veterans Administration has, the program would not exist. We simply wouldn't allow it. After all, you are only citizens, and we write your laws for you.
Thursday, August 25, 2005
Constant Gardener
I'm not sure how the book escaped my attention before now, since I have a growing library of anti-Pharma tomes. But I enjoyed it much more than Kakutani did. But whenever a reviewer complains that a book "devolves into an altogether conventional thriller" one should remember that the reviewer reads these things for a living. I don't, so the "conventional" part simply didn't apply. I reserve that sort of criticism for my Ebert moments, where I'm on surer footing.
The Author's Note at the end makes Le Carre's contempt for Pharma clear. He urges the reader to investigate a German outfit called BUKO Pharma-Kampagne, which is an independently financed Pharma watchdog group. Not in the Public Interest vein, but rather focused on the perfidy of Pharma's dealings in the developing world. Read about them here.
Tuesday, July 12, 2005
You're sick! You Spend too Much for it! You get little!
...as a consequence of our legal environment, drug companies have no choice but
to cram onto drug labels every adverse reaction reported, no matter how
rare....If a single adverse reaction has been reported and isn't included on the
label, the company will likely find itself on the losing end if sued by a
consumer who experiences that adverse reaction, on the theory that the company
''failed to warn'' of the remote risk of injury.
Sounds familiar, no?
You're sick!
What a great article that appears in the Seattle Times recently! As the introduction states,
Pharmaceutical firms have commandeered the process by which diseases are
defined. Many decision makers at the World Health Organization, the U.S.
National Institutes of Health and some of America's most prestigious medical
societies take money from the drug companies and then promote the industry's
agenda.
Damn right we have! Because we have the money to do it!
Check out the homepage of this excellent series, and spend some time reading the articles therein.
Friday, July 08, 2005
More ideas about DTC restrictions
Because I know my readers have too much free time (after all, how else did they find this site?) I would urge you to listen to this NPR program, "Justice Talking" which was an hour-long debate about drug approvals. The show aired last Spring, during Pharmablogger's hiatus. But nothing escapes my attention!
The debate was actually quite tame. Schering-Plough Chief Medical Officer Dr Bob Spiegel squared off against Dr Alastair Wood, of Vanderbilt and the NEJM. Dr Wood was far more reserved in his comments than Marcia Angell of Mr Goozner would have been. He defended profit motive as a driver for new drugs, and does not think they are approved too quickly. However, his remarks about post-marketing surveillance incentives were interesting. He would tie approval for Direct to Consumer (DTC) advertising, as well as exclusive formulary listing, to completion of Phase IV safety studies. He also acknowledges the 1st amendment issues, however. I thought this tied in nicely to the previous post about Bill Frist's DTC suggestions.
Economics and Health
Right on the heels of that article about pill-splitting being endorsed by insurers, we learn one of the largest pharmacy benefit managers, Express Scripts, has reported no cost increases from 2004 for companies and organizations that utilized a step program for prescriptions. The step programs consist of using older, less expensive and usually generic drugs before stepping up to newer, more expensive therapies. The article is from Managed Care Weekly, with no link available. No year-to-year cost increases? Hello, GM? Anyone home?
Tuesday, July 05, 2005
The Written Word, DIA, and WLF
I'll start this evening with an excerpt from the Wall Street Journal. I haven't a link, since it's a pay site. Suffice to say that it's the best newspaper in the world, and I highly recommend a subscription to anyone.
Amid recent worries that some impotence drugs could be associated with vision loss in rare cases, there were predictable suggestions that this information be added to the pills' instructions....But the directions, also called package inserts or labels, have become so bloated and poorly organized that
they often obscure the facts that would be most useful....A government
proposal to make the labels more useful has been in the works since 2000,
long before the recent controversies over safety problems with
antidepressants and arthritis drugs. ''We are working on a final rule now
and don't have a time frame at this point for its completion,'' an agency
spokeswoman said.
The notorious package insert. Long, tedious, meaningless? This article is important, because it clearly describes who the insert is written for - lawyers. It's a giant CYA, with contradictory messages. If the company neglects to put something in the label that they have data for, it's a warning defect, in legalese. But regardless of what they put in, they always preface the list of nasty effects with "regardless causality." So, we've warned you, but we're not saying if we think our drug did it. What a ridiculous method of trying communicate important information to physicians.
Frist vs the WLF?
Bill Frist has gone on record urging drug companies to refrain from DTC in the first two years of a drug's life in the market.
Frist warned that the flurry of consumer advertising that often accompanies
launches of new drugs can lead to inappropriate prescribing and unnecessary
health care spending. He said he is asking the Government Accountability Office
(GAO) to review the Food and Drug Administration's oversight of prescription
drug ads, the drug industry's drug ad spending, and its effect on drug
utilization and health care spending.
This is slightly astonishing, and quite welcome. The last Senator we heard discussing the dangers of DTC was Kennedy. Frist also questioned DTC's role in overprescribing, with deleterious health consequences. Clearly, Frist has wandered off the reservation here, as the article continues to give an opposing viewpoint from PHARMA. Again, no link here, as this was taken from an article from the Bureau of National Affairs, and you need an expensive subscription. DTC is absolutely huge in the first few months of a drug's lifecycle, because everyone is looking at the sales curve for the launch period. The sharpest uptick is always in the first year, and if you don't get the utilization you want during that period, you might never.
But wait, Bill - your Republican anti-trial lawyer free speech buddies probably don't agree with you! According the Washington Times, and the Washington Legal Foundation's own website, DDMAC is already violating the law when it comes to regulating the "free speech" of drug advertising. I admit that DDMAC can be somewhat capricious with these DTC matters. I've got a good story about that, for another time. But the WLF clearly wants to pave the way for drug companies to say whatever they want about their drugs, without any interference, as a matter of right under the First Amendment. I've said it before - my copy of the Constitution and Bill of Rights keeps talking about people, and doesn't contain the word "corporation" or "company" in it. In fact, there's only one reference to "property" at all, and it's the government's property. Where's a strict constructionist when you need one?
Saturday, June 25, 2005
An Autism retort, and BS at the WSJ
I don't wish to dwell on this issue long, but check out the New York Times today, with an examination of some of the personalities and seminal studies on autism. Near the end of the article, a researcher make the excellent point that regardless of the studies, we will know about the thimerosal-autism conclusively in two years, because that's when the first cases of post-thimerosal autism will be diagnosed. The question is, how many will there be?
Outrage of the Week
OOOOOOOOh, we're hot about this one. OpinionJournal online has a ridiculous screed from "a Boston Lawyer" (corporate defense, shall we assume?) about prosecutorial abuse of the US Attorney's office in Boston, against drug companies. Now that he's written a piece defending TAP, he's signed on board the Saddam Hussein defense team.
Seriously, though, there is no defense for TAP. They didn't even try to make one. In a typical bait-and-switch technique, he uses the acquittals of some unnamed TAP officials as vindication of the company as a whole. He also suggests that the qui tam payout of the whistleblower somehow undermines the legitimacy of the case. Fortunately, there's a public record here that can't be ignored - the US Attorney's settlement with TAP, with all of the salacious details of fraud contained therein. Remember, these guys cost you, the American taxpaper, hundreds of millions of dollars. I have a copy if you can't find it online - email me if you want it.
Here's a nice summary of qui tam suits, with specific information about the TAP and AstraZeneca cases, at cafepharma. We also found this article, by a defense attorney for one of the TAP officials, who also use the acquittals as a means to question the whole prosecution. What is especially appalling is that Pharmaceutical Executive would have even published his article. This is just the message we want our executives to hear, isn't it?
There's also mention of AstraZeneca in the OpinionJournal piece, which got in similar trouble with their Zoladex injections. Fortunately for them, their malfeasance was not nearly as grand (they actually had a legal department, unlike TAP, who referred to it as the "anti-profit" department). I haven't heard anyone at that company crying foul over prosecutorial zeal. I just wonder if all of the thousands of employees who now receive mandatory ethics training as a result of their company's settlement have any idea why that's happening.
Folks, fraud is fraud, regardless of what Medicare policies you object to, or how you feel about prosecutors in general. Don't try to change the facts by dressing up your objections in ideology.
Monday, June 20, 2005
An American Wannsee?
If you've been wondering why I haven't written about the Autism and Thimerosal article in last Thursday's Salon.com, wonder no further. I've been taking the time to research the article, so I could comment competently.
And what I've found is disappointing, given the topic and the article's hype.
Read it here, rather than Salon, so you don't have to sit through a commercial. Your first impression may match mine - a conspiratorial approach that reads like a paranoid thriller. But you can read the original conference transcript here for yourself.
What? You don't want to read a 268 page .pdf file? Why not??
Fine - here's what I found. Some of what RFK Jr wrote is simply bunk. This was a large conference, over 50 participants, held in run-of-the-mill conference center a few miles outside of Atlanta, because that's where the Centers for Disease Control and Prevention (CDC) is. There was a technology conference in the city, which apparently occupied all of the available space there, according to the organizers.
No notes or materials allowed to leave? Nonsense. Check out the request from one of the participants to get copies of slides for everyone, and the half-hearted request at the end for everyone to embargo their materials until the end of that month, when they would be made public.
But the most telling thing about this transcript is simply that it has been available on the web since November 2003. All of the other "references" that RFK Jr make are out there as well, some of it years old. The quotes that he picked out are the same quotes from an article at momsonamissionforautism.org, also dated 2003. Search for the quotes, particularly the "plaintiff" ones in the .pdf file, and you'll find that the participants who spoke them were hardly being surreptitious.
This was an RFK clip job, and not a good one.
Why oh why did he have to write this? Sure, the democraticunderground.com loves it, but this should have been written some time ago, by a real journalist, in somewhat less inflammatory tones.
Why? Because the story itself is real. Epidemiology matters! (What a great bumper sticker that would make!)
As my friends at Breast Cancer Action like to point out, the quest for cures is only part of the story, and perhaps not even the most compelling. But the rising incidence of autism has been so ridiculously dramatic over the last 15 years, that no one involved has missed it. This point is missed by the pink ribbon bunch, who ignore the doubling of breast cancer incidence in the last few decades, but that's another story.
I've blasted this article here, but I cannot urge you enough to read more on this topic. You may have read that Bill Frist is trying to attach a rider to a homeland security bill to give legal immunity to vaccine manufacturers, or that ABC tanked a story on RFK and Autism. I suppose if his article gains the issue some attention, than it's all for the good. Again, I just wish a real journalist, who knows how to attribute, had written it first.
Friday, June 17, 2005
Some fun this evening
The Future of Pharmaceutical Marketing?
An advert for this product appeared one recent Sunday morning in my paper, and I almost choked on my Hazelnut decaf.

I had fun calling a friend who works for McNeil Consumer Products, and he confirmed that the company is sliding down a steep cliff of intellectual bankruptcy. This is the company that brought us Tylenol ER, which was a coat-core Tylenol that lasted eight hours. It didn't sell, but their market research told them that anything with the word "Arthritis" in it would. So the same product was relaunched as "Tylenol Arthritis." The results were spectacular.
With flavored medication as inspiration, I had a few new ideas of my own. I wonder if they might take hold?

The Zoloft Happy Meal!

Some Ambien in your decaffeinated soda?
Thursday, June 16, 2005
Idiocy at CBS!
Against What Grain?
First, start with this article from CBS News writer Dick Meyer, published in February.
The article was sent to us from a well-meaning colleague at work, who was excited to read something positive about pharmas. We almost blew a gasket reading this. No, we DID blow a gasket, and replied to our colleague with the following verbatim text, copying the four others in our department who also received the initial email:
My impressions of the article, blog-style.
"We expect drug companies to be altruistic, not to be motivated by profits...I don't think they are more or less greedy or corrupt than companies in any other sector of the economy."
Why would we expect drug companies to be altruistic, a cut above other types of corporations? Because that's the image they are giving us, through their company branding adverts. They are actively selling themselves as the deliverers of a better life, with mission statements that sound like something written for the WHO. In fact, they have a fiduciary duty to maximize return to shareholders above all else, a point that is painfully clear to AstraZeneca, considering the wave of class-action suits being filed against us: see http://www.lerachlaw.com/cases/astrazeneca/complaint.pdf
"I don't think they are more or less greedy or corrupt than companies in any other sector of the economy...And just because they make medicine, their malfeasances are not quantitively more evil than those of other corporate evildoers."
Is this guy kidding? Pharma malfeasance kills people. How many people died as a result of Enron, Global Crossing, MCI/Worldcom, Arthur Andersen, etc?
"Personally, I am incredibly thankful for drug companies. People I care about deeply are alive and full-strength because of drug companies."
How about some recognition for the NIH or NCI? Others have spent many electrons and killed many trees discussing Pharma perfidy in claiming medical advances; I shall not attempt to duplicate them here. One example only: http://lists.essential.org/pipermail/pharm-policy/2000-June/000215.html
"When more people use more effective drugs, overall health spending costs are lessened and national productivity goes up enormously, but in incalculable ways." Correct. But what is being prescribed? The ALLHAT trial http://allhat.sph.uth.tmc.edu/ clearly demonstrated the wastefulness of current prescribing habits for hypertension, to give one example. I have personal experience with this - when my wife was initially diagnosed with postpartum hypertension, our family doc reached for his prescription pad, and gave her a script for a (under patent) calcium channel blocker. We "fired" that pharma bimbo asap. For something closer to home, see: http://www.prescriptionaccesslitigation.org/resource.php?doc_id=662
"Critics don't want drug companies to advertise like other companies. They say the money should be spent to make drugs cheaper, which is a bogus point."
Classic strawman argument. How the heck does this guy know what critics are thinking? Basic economics shows that (successful) DTC doesn't raise the price of individual drugs - it pays for itself through higher sales volume. But at what price? Are the most effective treatments being advertised, or just the newest and most expensive? Are physicians prepared to discuss with their patients why they prefer to prescribe X instead of Y, when the patient insists on Y because she saw a lady with arthritis dancing around on TV? See: http://www.jabfp.org/cgi/content/full/16/6/513. - from study article: "Physicians filled 69% of requests they deemed clinically inappropriate"
"When a patient uses an expensive prescription drug for heartburn when a cheap over-the-counter might work just as well, we blame the drug company. But isn't the doctor more or equally responsible?"
Absolutely the physician is partly responsible, but they work within an irrational system. They continue to prescribe Nexium when Prilosec OTC is just as good, because they know that the insurance won't pay for the OTC.
"But the decisions about how medical care is distributed in this country are our decisions, collectively, through elections, laws and government...It is only by a spectacular feat of cynicism that our political system's moral negligence has become the fault of the pharmaceutical industry," Gladwell wrote in 'The New Yorker.'"
Again, is this guy for real? An investigative journalist? Has he read the Medicare Modernization Act, and seen the provisions that were inserted DIRECTLY FOR PHARMA that prohibit price competition, like the VHA enjoys (again, only one example)? Does any other industry have the benefits bestowed upon pharma by Congress? Has he checked to see what industry is the largest political contributor in the US?
Damn, we loved that reply! We wrote it all in a fury, with the links, in about 15 minutes. After we sent it, we panicked, and followed-up with another email begging the recipients to PLEASE not forward the message to anyone else. Brilliant analysis, perhaps, but lousy judgment. We also sent a copy to Dick Meyer, who replied with a polite Thank You. We had every intention of blogging this article at the time, but events intervened.
Save those links. Impress your friends and relations. Ciao.
Tuesday, June 14, 2005
Ethical Blinders
Book report project
Today we start off with an assignment for a new book that doesn't quite cast the drug company in such demonic lights, as have our previous assignments. It's more illustrative of the powerful personalities who dwell at the summit of medical research and business. Mild personalities simply don't reach these spectacular heights, which explains why we are plugging away at this blog anonymously, while we should be doing other mundane tasks.
Such a clash is illustrated in a new book, reviewed here in the Times, describing how one alleged scandal over drug safety morphed into an altogether different affair surrounding the principal investigator for a Canadian drug, Nancy Olivieri (described as "charismatic"), her attempts to whistleblow the company supporting her, and why it may have all been smoke and mirrors. Not available in the US yet, so far as we can tell.
Pill splitting backed by insurers
The Associated Press has a interesting story, found in today's Philadelphia Inquirer, about how pill splitting to save money is being encouraged by insurers. If multiple dose forms are priced similarly, and there are no issues with instability or coat-core formulations, why not get a higher prescription strength Lipitor than you need, and cut it in half?
Ethical blinders?
It was the last sentence in this article that grabbed our attention. We got most of the way through this thinking that a CME "wall of separation" between pharmas and docs may be possible someday, if these trends continue. But then the Cephalon spokeswoman has to go ahead and say something stupid like "...it's an opportunity for us as a company to start building a presence in psychiatry" while discussing the psychiatry course they are underwriting.
If she's reading this (hah!), she probably has no idea what we are objecting to. Therein lies the problem.
We've had many a conversation with sales folks (who have a much more difficult job than we) about influence over physician prescribing habits. VERY little is required to make one person feel some kind of indebtedness to another, when it comes to gift giving. Pharmas know that well. Even the merest office trinkets, or under $25 "lunch and learns" can influence a doc to mke a decision they would not ordinarily make. See this link for the statistics involved. The reps we have known talk about building their relationship with physicians, often becoming quite chummy. And the kicker - they honestly, in their gut, think that is perfectly OK. Why not? Salesmen from other industries certainly get that way with clients.
Do we need to write more, or are you getting it? Would you like to know all of the factors that went into the decision for your doc to write you an Rx for drug xyz? And would you be comfortable knowing that the sales "relationship" was a factor, however background it may be?
The reps, the Cephalon spokesbabe, the docs - all with moral blinders. Is it immoral when they don't even see the conflict? Why is anything besides science guiding medical decision-making here? After all, every company claims that each of their products is the superior product for clinical reasons. Let's make them back that up without the relationships.
Tomorrow: The article that almost brought us out of retirement last February.
Thursday, June 09, 2005
Pharmablogger Returns!
The news is just too rich too stay away. Let's start with our friend from Pfizer, Veep Peter Rost, recently profiled in this New York Times piece. It seems that Dr Rost is given precious little to do these days at Pfizer, after mouthing off about the truth of pharmaceutical marketing. He even reads some of the same books that we do! So why not fire him? Well, there's the possibility of Pfizer violating an anti-whistleblower statute.... Read the piece for more. Today. It's already a few days old, and the Times doesn't let you access the articles for very long.
Then we have our always reliable friends at Merck, who are responsible for two great pieces relating to Vioxx, and the pressure they place on researchers not to speak evil of their blockbuster. Start with this article from the Philadelphia Inquirer to get a taste of what kind of pressure they can exert. Than get a fuller picture from this piece from NPR's All Things Considered.
NPR got hold of documents that were obtained through some kind of discovery process at Merck. The New York times has done the same for Johnson & Johnson, to write this article about Propulsid. Let's focus on this sentence: "An internal company memo examined 15 of the proposed label changes and estimated that they would cost over $250 million a year in lost sales." That's just for a label change that many doctors will ignore anyway, continuing to prescribe off-label for the kiddies. No more need be said there.
There is a reference in the article to a film called "MAMA/M.A.M.A.," by Nonny de la Peña, which we have not seen. It's apparently about Munchausen Syndrome by Proxy disease. As a service, we will look into this film more. That's a particularly bizarre syndrome that we've read about in MedWatch reports for certain psychoactive meds we work with.
But we digress. After reading the Inquirer piece, like always our reaction began with "How appalling!" to "Gee, we wonder if we've ever done that?" Frankly, we have no idea. And no belief that we would ever find the truth about our company, absent a lawsuit with leaked documents.
Wednesday, January 05, 2005
Malpractice shield redux
Today's Houston Chronicle discusses how Vioxx lawsuits in Texas may likely go nowhere, thanks to year-old tort reform legislation that protects drug companies "...as they can prove that any warnings of harmful side effects were approved by the Food & Drug Administration."
"To win against Merck in Texas, Vioxx plaintiffs will have to prove that the company withheld information or misrepresented it to the government. That will make litigation so expensive, some lawyers said, that they will accept only serious cases, in which plaintiffs were badly harmed and damage awards could be high. "
There is a possibility of combining the hundreds or thousands of Texas suits with suits from other states by the federal Judicial Panel on Multidistrict Litigation in Washington. This may be in Merck's favor however, as the litigation will become increasingly complex, and timelines will be stretched out for years. Remember, corporations may be "legal artificial persons" under the law, but they have the distinct advantage of not needing to age or die, unlike many of the "natural person" plaintiffs.
Here is a Law.com article about the other state with this protection, Michigan. Their law was passed in 1995, and withstood appeals to the Michigan Supreme Court.
"Jim O'Reilly, law professor at the University of Cincinnati and author of treatises on the FDA and product warnings, said the Michigan statute is unusual because states do not usually cede power to the federal government without pre-emption. A former associate general counsel at Procter & Gamble, O'Reilly added, 'the state is deciding to give away the rights of the individual" so that career federal agency employees in Washington "are now exercising the delegated power of a jury.'"
Jeff Trewhitt, spokesdude for PHRMA says that "the association has long supported an "FDA defense" to punitive damages if a drug company can prove that it followed all of the agency's rules and regulations in good faith." But frankly, even this is crap. The reality is that drug companies have to prove nothing. The plaintiffs will have to go through ridiculous amounts of discovery to prove that the drug companies did not follow regs in good faith. Do you know how much meaningless paper and garbled statistics a company can dump on a plaintiff to make discovery an impossible journey? We do. We've been instructed to hold all papers, emails, etc. with the name of certain drugs on it, in case of expected future litigation. We'll be able to bury any plaintiff with mountains of paper, should they dare to ask for it.
In today's mandatory Washington Post article on the subject of tort reform, pinhead "Victor E. Schwartz of the American Tort Reform Association. "If you have done everything the law requires, why should you be punished?" Victor missed the part in Torts class concerning the Common Law, and that regulations make up a tiny amount of what we call "the law." Let's just stuff the regulatory agencies with business people who don't care about patients or consumers, and then give those agencies the burden of deciding when the businesses subject to their regs can or cannot be found liable.
No thanks. The jury system can be found in the Constitution, while FDA deciding what is legally liable is not. Just call me a strict constructionist!
Monday, January 03, 2005
Robert Reich is ahead of Pharmahost
For a different tale of Drug Safety, we offer you a link to an article in The New Yorker concerning the testing of drug in pediatric populations. The article is long, and makes several important points. We would like to focus on the idea that any drug under consideration for approval should be highly scrutinized in vulnerable populations for safety, and indeed we have seen this for the hepatically impaired in recent years. Liver failure has been one of the most common reasons for drug withdrawals or non-approvals (see Duract and Exanta). The Duract example is good because no one could get doctors to stop over-prescribing that drug. You have to wonder, does a company in that position really try to get a warning message across, right down at the sales rep level, even when the FDA is breathing down their necks?
Anyway, we would expect that real pediatric studies would be expected for whoever brings forth the next anti-depressant, based on recent bad publicity of Paxil and other SSRIs (studies in which no one actually committed suicide, but had events coded to the term "suicidal ideation"). This is still a scattershot approach, however. Scrutiny will only be brought to bear on the categories of drugs that have already been demonstrated to be a hazard.
Lastly, for our daily dose of re-importation nonsense, see this article from CIPA, a Canadian mail-order pharmacy group, that contends President Bush is behind a new effort by the Health Ministry to shut down shipments to US addresses. We love the way they make us sound like an underprivileged country in this bit:
"Dosanjh (health minister) drafted the new regulations that, if implemented,will make it illegal for Canadian mail-order pharmacies to fill prescriptions for non-citizens, and leave millions of Americans without access to affordable medications."
Oh wait, I forgot. For health care coverage, we ARE a third world country.